Around 800 of the about 23,000 qualities thought to involve the human genome code for G protein-coupled receptors. Albeit a significant number of these are odorant receptors in olfactory neurons, numerous others are receptors for all around portrayed neurotrans-mitters utilized all through the sensory system. Regardless of their tremendous variety, all G protein-coupled receptors comprise of a solitary polypeptide with seven trademark layer spreading over areas (serpentine receptors) G protein-coupled receptors contain seven film traversing spaces.
The β2-adrenergin receptor appeared here is illustrative of G protein-coupled receptors, including the β1-adrenergic and muscarinic acetylcholine (ACh) receptors and rhodopsin. It comprises of a solitary subunit with an extracellular amino end, intracellular carboxy end, and seven film spreading over α-helixes. The coupling site for the synapse lies in a parted in the receptor framed by the transmembrane helixes. The amino corrosive buildup aspartic corrosive (Asp)- 113 partakes in authoritative.
The piece of the receptor demonstrated in earthy colored partners with G protein α-subunits. Two serine (Ser) deposits in the intracellular carboxy-terminal tail are locales for phosphorylation by explicit receptor kinases, which inactivates the receptor. (Adjusted, with authorization, from Frielle et al. 1989.)
The quantity of substances that go about as second couriers in synaptic transmission is many less than the quantity of transmitters. Around 100 substances fill in as transmitters; each can initiate a few sorts of receptors on the cell surface. The couple of second couriers that have been all around portrayed fall into two classifications, intracellular and transcellular.
Intra-cell couriers are particles whose activities are restricted to the cell in which they are created. Trans-cell couriers are particles that can promptly cross the phone layer and hence can leave the phone wherein they are delivered to go about as intercellular signs, or first couriers, on neighboring cells.
A Family of G Proteins Activates Distinct Second-Messenger Pathways
The primary G protein, Gs (where “s” represents stimulatory), was distinguished over 30 years back by Martin Rodbell, Al Gilman, and their associates. Since that time a huge group of G proteins has been distinguished. The G proteins are related with the internal pamphlet of the plasma layer, where they communicate with G protein-coupled receptors.
The G proteins that couple receptor actuation to intracellular effectors are trimers that comprise of three subunits: α, β, and γ . The α-subunit is just inexactly connected with the film and is normally the operator that couples the receptor to its essential effector compound. The β-and γ-subunits structure an emphatically bound complex that is all the more firmly connected with the film. As we will learn later in this part, the βγ complex of G proteins can likewise manage the action of certain particle channels straightforwardly. Roughly 20 sorts of α-subunits have been recognized, 5 kinds of α-subunits, and 12 sorts of γ-subunits.
G proteins with various α-subunits couple various classes of receptors and effectors, and accordingly have distinctive physiological activities. The β-adrenergic receptor initiates adenylyl cyclase by following up on Gs proteins; these contain the αs sort of α-subunit. A few mus-carinic ACh receptors restrain the cyclase by following up on Gi proteins (where “I” represents inhibitory); these contain the αi type subunit. Then again other G proteins (Gq/11 proteins, which contain αq – or α11-subunits) initiate phospholipase C and presumably other sign transduction systems not yet distinguished.
The Go protein, which contains the αo-subunit, is communicated at especially elevated levels in the mind. Contrasted and different organs of the body, the cerebrum contains an uncommonly enormous assortment of G proteins. All things considered, as a result of the predetermined number of classes of G proteins, one sort of G protein can frequently be enacted by various classes of receptors.
The realized effector focuses for G proteins are more restricted than the sorts of G proteins. Significant effectors incorporate certain particle channels that are initiated by the βγ complex, adenylyl cyclase in the cAMP pathway, phospholipase C in the diacylglycerol-inositol polyphosphate pathway, and phospholipase A2 in the arachidonic corrosive pathway.
Every one of these effectors (aside from the particle channels) starts changes in explicit objective proteins inside the cell, either by producing second couriers that quandary to the objective protein or by enacting a protein kinase that phosphorylates it. Notwithstanding their disparities, second-courier pathways enacted by G protein flagging offer a typical plan.
Synaptic second-courier frameworks including G protein coupling follow a typical succession. The sign transduction pathways represented here include comparable advances (left). Substance transmitters showing up at receptor atoms in the plasma film enact a firmly related group of G proteins (the transducers) that actuate various compounds or channels (the essential effectors). The enacted chemicals produce a second courier that initiates an auxiliary effector or acts straightforwardly on an objective (or administrative) protein. cAMP framework.
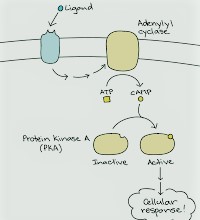
This pathway can be enacted by a transmitter-bound β-adrenergic receptor, which acts through the Gs protein αs-subunit to initiate adenylyl cyclase. Adenylyl cyclase produces the subsequent courier cAMP, which actuates PKA. The G protein here is named Gs since it invigorates the cyclase. A few receptors actuate a G! protein that represses the cyclase. Phosphoinositol framework. This pathway, initiated by a sort 1 muscarinic acetylcholine (ACh) receptor, utilizes the Gq or G11 kind of G protein (with α or α11-subunits, separately) to actuate an essential effector, phospholipase Cβ (PLCβ).
This protein hydrolyzes the phospholipid, phosphatidylinositol 4,5-bisphosphate (PIP2), yielding a couple of second couriers: diacylglycerol (DAG) and inositol 1,4,5-trisphosphate (IP3). Thusly, IP3 discharges Ca2+ from interior stores, while DAG enacts protein kinase C (PKC). The drop in layer PIP2 levels can straightforwardly adjust the movement of some particle channels.
Direct G protein-gating.:This pathway speaks to the least complex synaptic system for G protein-coupled receptor activity. Acetylcholine (ACh) following up on type 2 muscarinic receptors enacts the Gi protein, prompting practical separation of the α-subunit and βγ complex. The βγ complex associates straightforwardly with a G protein-gated internal amending K+ channel (GIRK), prompting channel opening and layer hyperpolarization.
Hydrolysis of Phospholipids by Phospholipase C Produces Two Important Second Messengers, IP3 and Diacylglycerol.Numerous significant second couriers are produced through the hydrolysis of phospholipids in the inward pamphlet of the plasma layer. This hydrolysis is catalyzed by three compounds—phospholipase C, phospholipase D, and phospholipase A2—named for the ester bonds they hydrolyze in the phospholipid.
The phospholipases each can be actuated by various G proteins coupled to various receptors. The most generally hydrolyzed phospholipid is phosphatidylinositol 4,5-bisphosphate (PIP2), which ordinarily contains the unsaturated fat stearate esterified to the glycerol spine in the principal position and the unsaturated fat arachidonate in the second:
Enactment of receptors coupled to Gq or G11 animates phospholipase C, which prompts the hydrolysis of PIP2 (explicitly the phosphodiester bond that connects the glycerol spine to the polar head gathering) and creation of two second couriers, diacylglycerol (DAG) and inositol 1,4,5-trisphosphate (IP3). DAG, which is hydrophobic, stays in the film when framed, where it selects the cytoplasmic protein kinase C (PKC). PKC and DAG along with certain film phospholipids structure a functioning complex that can phosphorylate numerous protein substrates in the cell, both layer related and cytoplasmic
Enactment of some isoforms of PKC requires raised degrees of cytoplasmic Ca2+ notwithstanding DAG.
Hydrolysis of phospholipids in the cell layer initiates three significant second-courier falls.
A. The authoritative of transmitter to a receptor actuates a G protein that initiates phospholipase Cβ (PLCβ). This chemical severs phosphatidylinositol 4,5-bisphosphate (PIP2) into the subsequent couriers, inositol 1,4,5-trisphosphate (IP3) and diacylglycerol (DAG). IP3 is water-dissolvable and diffuses into the cytoplasm, where it ties to a receptor-channel on the smooth endoplasmic reticulum, the IP3 receptor, to deliver Ca2+ from inward stores.
DAG stays in the layer, where it enacts protein kinase C (PKC). Layer phospholipid is additionally an important cofactor for PKC actuation. Some isoforms of PKC additionally require Ca2+ for initiation. PKC is made out of a solitary protein particle that has both an administrative space that ties DAG and a reactant area that phosphorylates proteins on serine or threonine buildups.
B. The Ca2+/calmodulin-subordinate protein kinase is enacted when Ca2+ ties to calmodulin. The Ca2+/calmodulin complex at that point ties to an administrative area of the kinase, causing its enactment. The kinase is made out of numerous comparative subunits (just one of which is appeared here), each having both administrative and synergist capacities. The reactant space phosphorylates proteins on serine or threonine deposits. (ATP, adenosine triphosphate; C, reactant subunit; COOH, carboxy end; H2N, amino end; R, administrative subunit.)
The second result of the phospholipase C pathway, IP3, invigorates the arrival of Ca2+ from intracellular layer stores in the lumen of the smooth endoplasmic reticulum. The layer of the reticu-lum contains an enormous indispensable film macromol-ecule, the IP3 receptor, which structures both a receptor for IP3 on its cytoplasmic surface and a C










Nicely written
Good article
Nicely written
Good article