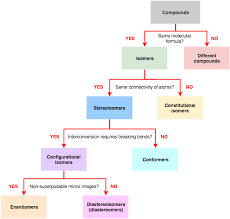
The three-dimensional spatial game plan of the iotas of an atom is named its stereochemistry. A particle might be distinguished first by its compound recipe, then, at that point by its synthetic design, lastly by its atomic design. For instance, is the substance recipe of ethyl liquor. is the substance design of ethyl liquor. The atomic construction is dictated by the three dimensional plan of the iotas in space. The word ‘design’ is utilized to depict the particular game plan of different molecules or gatherings appended to a chiral focus (as depicted underneath).
The word ‘compliance’ portrays the various plans of iotas that are gotten when parts of the particle are pivoted around one of the bonds. An adjustment of the setup fundamentally includes breaking somewhere around one covalent bond and changing it after a revamp of the iotas. A change in the adaptation fundamentally doesn’t include any breaking of covalent securities. Gatherings that are associated by a solitary bond can go through turn prompting diverse relative directions with deference to one another. For instance, in ethyl liquor, contingent upon the point of revolution about the C–C bond, the general directions of hydrogen particles and the hydoxyl gathering will differ. Enthusiastically, the most supported compliance about any bond is the ‘amazed’ one wherein molecules connected at one or the flip side of the bond are at the biggest conceivable separation from one another.
An ‘overshadowed’ conformity increments steric contacts and is significantly less good. Courses of action of particles or gatherings showed up at by turn around a twofold bond are configurational isomers not conformational isomers. This is on the grounds that the twofold bond doesn’t permit free revolution. To deliver the isomers, it turns out to be thusly important to break the twofold bond and afterward change it after the modification of the particles. cis-trans isomers fall under this classification and are seen on account of the amino corrosive proline and in polypeptides.
Direct polypeptides comprise solely of trans peptide units while cyclic peptides can have the two cis what’s more, trans peptide units. In certain particles the distinctions in the plan of the iotas may not influence a large number of the actual properties of the atom like softening point, thickness, refractive list, and so forth, however may influence the collaboration of the various atoms with energized light. Such particles are supposed to be optically dynamic and are called optical isomers. Optically dynamic particles turn in various ways the plane of polarization of the plane captivated light occurrence on them. Levo-rotatory atoms pivot the plane the clockwise way and dextro-rotatory atoms pivot it in the anticlockwise course. These optically dynamic atoms might vary in their organic conduct.
For instance, just one of the optical isomers of aspargine is sweet to taste. Louis Pasteur showed that the distinction in real life between two optical isomers is because of the distinction in sub-atomic design, for example due to contrasts in the plan of the iotas in space. The two kinds of particles are connected by a reflect reflection and they can’t be superimposed. Objects of regular daily existence which are enantiomers (for example related by reflect balance) are the right hand and the left hand, righthanded and lefthanded screws, and so forth The two alanine particles displayed in Figure 1.9 have similar number of iotas however contrast in their stereochemistry and subsequently are known as stereoisomers. Mixtures with four unique replacements at a carbon molecule will display optical movement. Such a carbon molecule is for the most part known as a hilter kilter carbon and a particle containing such an iota is a chiral atom. A combination of two isomers, which have optical movement inverse to one another, is all in all optically idle since the impact of one is invalidated by the other. Such a blend is known as a racemic combination.
A couple of enantiomeric particles will act similarly regarding even conditions or achiral synthetic reagents. Nonetheless, their cooperation with other chiral particles will contrast. An illustration of this is the unmistakable cooperation between a protein and its substrate. Stereoisomers not related by reflect evenness are called diastereoisomers. Diastereoisomerism will happen in particles with more than one chiral focus. Both configurational and conformational contrasts can prompt optical isomers—for example either enantiomers or diastereoisomers. Other than carbon, particles like silicon with a valency of four can likewise go about as chiral focuses. A few particles like hexahelicene, also, polyglycine show optical movement however they have no chiral focus. This is expected to the helical structures expected by these atoms, which can be either correct given or left gave.
Every one of the major organic particles, similar to amino acids, proteins, DNA, sugars and lipids, are optically dynamic. Out of the at least two optical isomers accessible for these atoms only one kind is ordinarily found in organic frameworks. Optical action and Life appear to be indivisible.
Stereochemical classification
Particles are three-dimensional substances. To address them in two measurements, for example on a piece of paper some standard techniques are accessible. The most generally utilized are the Newman projection and the
Fischer projection
What’s more, there is one more show used to depict the game plans of different gatherings around a chiral focus. This documentation is known as the Cahn-IngoldPrelog framework and the enantiomers are known as the [R] and the [S] arrangements. The R–S show is as per the following: If the gatherings joined to the chiral focus are orchestrated by the arrangement rules (given beneath) as then, at that point if the chiral focus is seen from the side inverse to the most minimal gathering (for example d for this situation), and if happen a clockwise way, the atom is in the [R] design. Then again on the off chance that they happen the counter clockwise way, it is in the [S] setup .
The succession rules are as per the following:
(1) Atoms are organized agreeing to nuclear number, for example C1 > O > N > C > H.
(2) If the joined particles are something similar, then, at that point the following molecule is utilized for requesting, for example
(3) If the substituents have molecules of a similar position then the one with more particles of the greatest position gets inclination, for example also, etc.
Since at each chiral molecule there are two manners by which the joined gatherings can be masterminded, if there are n chiral focuses then there will be stereoisomers. On account of amino acids an alternate documentation is utilized. In this particle the molecule is the chiral focus. In the event that we peer down the bond and assuming is perused the clockwise way, it is the L arrangement. In the event that it is perused the counter clockwise way, it is the D setup. All normally happening amino acids are in the L setup
© 2021 Niazi TV – Education, News & Entertainment