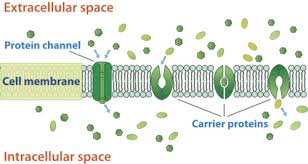
These systems of development are known as transporter intervened transport. Every transporter protein is intended to perceive just a single substance or one gathering of fundamentally the same as substances. Exploration has connected imperfections in explicit transporter proteins with explicit illnesses.
A film transport protein (or basically carrier) is a layer protein that goes about as such a transporter. A vesicular vehicle protein is a transmembrane or layer related protein. It directs or encourages the development by vesicles of the substance of the cell.
Distinction Among Channels and Transporters
A transporter isn’t open all the while to both the extracellular and intracellular conditions. Either its inward door is open, or external entryway is open. Conversely, a channel can be available to the two conditions simultaneously, permitting the particles to diffuse without interference. Transporters have restricting locales, however pores and channels don’t.
At the point when a channel is opened, a huge number of particles can go through the layer every second, except just 100 to 1000 atoms normally go through a transporter atom in a similar time. Every transporter protein is intended to perceive just a single substance or one gathering of fundamentally the same as substances. Exploration has related imperfections in explicit transporter proteins with explicit infections.
Dynamic Vehicle
The activity of the sodium-potassium siphon is a case of essential dynamic vehicle. The two transporter proteins on the avoided are utilizing ATP to move sodium with regards to the cell against the fixation angle. The proteins on the privilege are utilizing auxiliary dynamic vehicle to move potassium into the cell.
Dynamic vehicle is the development of a substance over a layer against its fixation inclination. This is generally to gather high centralizations of atoms that a cell needs, Glucose or amino acids, for example. In the event that the cycle uses synthetic energy, such as adenosine triphosphate ( ATP), it is called the essential dynamic vehicle. Optional dynamic vehicle involves the use of an electrochemical inclination and does not use the energy generated in the cell.
Not at all like channel proteins which just vehicle substances through films inactively, transporter proteins can move particles and atoms either latently through encouraged dispersion, or by means of optional dynamic vehicle. A transporter protein is needed to move particles from regions of low focus to territories of high fixation.
These transporter proteins have receptors that dilemma to a particular atom (substrate) requiring transport. The particle or particle to be moved (the substrate) should initially tie at a coupling site at the transporter atom, with a specific restricting fondness. Following authoritative, and keeping in mind that the coupling site is confronting a similar way, the transporter will catch or impede (take in and hold) the substrate inside its atomic structure and cause an inner movement so the opening in the protein currently faces the opposite side of the plasma film. The transporter protein substrate is delivered at that site, as per its coupling partiality there.
Encouraged Dissemination.
Encouraged dissemination is the entry of particles or particles over an organic layer through explicit vehicle proteins and requires no energy input. Encouraged dispersion is utilized particularly on account of huge polar particles and charged particles; when such particles are disintegrated in water they can’t diffuse openly across cell films because of the hydrophobic idea of the unsaturated fat tails of the phospholipids that make up the bilayers.
The kind of transporter proteins utilized in encouraged dissemination is somewhat unique in relation to those utilized in dynamic vehicle. They are still transmembrane transporter proteins, yet these are gated transmembrane channels, which means they don’t inside move, nor expect ATP to work. The substrate is taken in one side of the gated transporter, and without utilizing ATP the substrate is delivered into the cell. They might be utilized as possible biomarkers
Switch Transport
Switch transport, or carrier inversion, is a marvel wherein the substrates of a film transport protein are moved the other way to that of their normal development by the transporter. Transporter inversion commonly happens when a layer transport protein is phosphorylated by a specific protein kinase, which is a chemical that adds a phosphate gathering to proteins.
Types
1: Channels/pores
1. α-helical protein channels, for example, voltage-gated particle channel (VIC), ligand-gated particle channels(LGICs)
2. β-barrel porins, for example, aquaporin
3. channel-framing poisons, including colicins, diphtheria poison, and others
4. Nonribosomally blended channels, for example, gramicidin
5. Holins; which work in fare of proteins that digest bacterial cell dividers in an early advance of cell lysis.
Encouraged dispersion happens all through the cell film by means of channels/pores and transporters/doormen.
Channels:
Directs are either in open state or shut state. At the point when a channel is opened with a slight conformational switch, it is available to both climate all the while (extracellular and intracellular)
This image speaks of symptoms. The yellow triangle shows the angle of focus for the yellow circles, while the green triangle shows the angle of fixation for the green circles, and the purple poles are the vehicle protein pack. The green circles move against their angle of fixation by means of a vehicle protein that requires energy, while the yellow circles lower their focal inclination, which discharges energy.
By chemiosmosis, the yellow circles generate more energy than is needed to move the green circles so that the growth is coupled and some energy is counterbalanced. The lactose permease, which allows protons to go down the focus slope into the cell while also syphoning lactose into the cell, is one model.
Pores:
Pores are consistently open to these both climate, since they don’t go through conformational changes. They are consistently open and dynamic.
2: Electrochemical potential-driven carriers
2.A: Porters ( uniporters, symporters, antiporters), SLCs.
The image talks to Uniport. For the yellow circles, the yellow triangle shows the fixation slope and the purple bars are the vehicle protein pack. Since their fixation propensity is lowered by a vehicle protein, chemiosmosis can deliver energy. GLUT1, which moves glucose into the cell down its fixation slope, is one model.
Excitatory Amino Corrosive Carriers (EAATs)
EAAT1
EAAT2
EAAT3
EAAT4
EAAT5
Glucose carrier
Monoamine carriers, including:
Dopamine carrier (DAT)
Norepinephrine carrier (NET)
Serotonin carrier (SERT)
Vesicular monoamine carriers (VMAT)
Adenine nucleotide translocator (ANT)
2.B: Nonribosomally integrated watchmen, for example,
The (Nigericin) Family
The (Ionomycin) Family
2.C: Ion-inclination driven energizers
3: Primary dynamic carriers
3.A: P-P-bond-hydrolysis-driven carriers :
• ATP-restricting tape carrier (ABC carrier, for example, MDR, CFTR
• V-type ATPase ; ( “V” identified with vacuolar ).
• P-type ATPase ; ( “P” identified with phosphorylation, for example, :
Na+/K+-ATPase
Plasma film Ca2+ ATPase
Proton Siphon
This image speaks to antiport. The yellow triangle shows the fixation inclination for the yellow circles while the blue triangle shows the focus slope for the blue circles and the purple poles are the vehicle protein group. The blue circles are moving against their fixation angle through a vehicle protein which requires energy while the yellow circles descend their focus inclination which discharges energy.
The yellow circles produce more energy through chemiosmosis than what is needed to move the blue circles so the development is coupled and some energy is counteracted. One model is the sodium-proton exchanger which permits protons to go down their focus inclination into the cell while siphoning sodium out of the cell.
F-type ATPase; (“F” identified with factor), including: mitochondrial ATP synthase, chloroplast ATP synthase1
• 3.B: Decarboxylation-driven carriers
• 3.C: Methyltransfer-driven carriers
• 3.D:Oxidoreduction-driven carriers
• 3.E:Light absorption-driven carriers, for example, rhodopsin
4: Group Translocators
The gathering translocators give an extraordinary component to the phosphorylation of sugars as they are moved into microscopic organisms (PEP bunch movement)
5: Electron transporters
The transmembrane electron move transporters in the film incorporate two-electron transporters, for example, the disulfide security oxidoreductases (DsbB and DsbD in E. coli) just as one-electron transporters, for example, NADPH oxidase. Frequently these redox proteins are not viewed as transport proteins.
Models
Every transporter protein, even inside a similar cell film, is explicit to one kind or group of atoms. For instance, GLUT1 is a named transporter protein found in practically all creature cell layers that transports glucose over the bilayer. Other explicit transporter proteins likewise help the body work in significant manners. Cytochromes work in the electron transport chain as transporter proteins for electrons.
Pathology
Various acquired infections include absconds in transporter proteins in a specific substance or gathering of cells. Cysteinuria (cysteine in the pee and the bladder) is such an illness including inadequate cysteine transporter proteins in the kidney cell films. This vehicle framework regularly eliminates cysteine from the liquid bound to become pee and returns this fundamental amino corrosive to the blood. At the point when this transporter breakdowns, huge amounts of cysteine stay in the pee, where it is moderately insoluble and will in general accelerate.
This is one reason for urinary stones. Some nutrient transporter proteins have been demonstrated to be overexpressed in patients with threatening sickness. For instance, levels of riboflavin transporter protein (RCP) have been demonstrated to be essentially raised in individuals with bosom disease