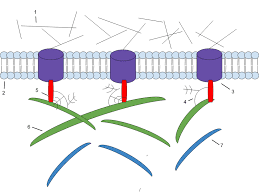
Integrins.
The integrin family includes heterodimeric vital film proteins that work as bond receptors, interceding numerous cell–network connections. In vertebrates, at any rate 24 integrin heterodimers, made out of 18 kinds of alpha subunits and 8 sorts of beta subunits in different mixes, are known.
A solitary beta chain can cooperate with any of various alpha chains, framing integrins that predicament various ligands. Portions of both the alpha and the beta subunits of an integrin atom add to the essential extracellular ligand restricting site.
Ligand authoritative to integrins likewise requires the concurrent official of divalent cations (emphatically charged particles).
The individuals from this huge family have a momentous capacity to transmit flags in the two headings over the cell film. The official of a network part to an integrin can communicate something specific into the inside of the phone, and conditions in the phone inside can impart a sign outward to control authoritative of the integrin to grid.
Capacities:
In epithelial cells integrins assumes a significant job in holding fast cells to grid in the basic basal lamina.
Some integrins, especially those communicated by certain platelets, take part in heterophilic cell–cell connections.
The individuals from this huge family assume significant jobs in grip and motioning in both epithelial and nonepithelial tissues.
In addition, the shortcoming of individual integrin-intervened connections encourages cell relocation.
Notwithstanding their grip work, integrins can intercede outside-in and back to front exchange of data (flagging). Integrin-interceded flagging pathways impact forms as different as cell endurance, cell multiplication, and modified cell demise.
Integrin-Containing Adhesive Structures Physically and Functionally Connect the ECM and Cytoskeleton in Nonepithelial Cells
Decent variety of Ligand–Integrin Interactions Contributes to Numerous Biological Processes
Cell–Matrix Adhesion Is Modulated by Changes in the Binding Activity and Numbers of Integrins
Collagen
The collagens, a group of sinewy proteins found in every multicellular creature. Collagens are discharged in huge amounts by connective tissue cells and in littler amounts by numerous other cell types. As a significant part of skin and bone, they are the most bottomless proteins in warm blooded animals, where they establish 2svo of the all out protein mass.
The essential component of a run of the mill collagen atom is its long, hardened, triple stranded helical structure, in which three collagen polypeptide chains, called alpha chains, are twisted around each other in a ropelike superhelix. Collagens are very wealthy in proline and glycine, the two of which are significant in the development of the triple-stranded helix.
Proline,
In light of its ring structure, settles the helical adaptation in every alpha chain, while glycine is routinely separated at each third buildup all through the focal area of the alpha chain. Being the littlest amino corrosive (having just a hydrogen particle as a side chain), glycine permits the three helical cx chains to pack firmly together to frame the last collagen superhelix.
The human genome contains 42 unmistakable qualities coding for various collagen alpha chains. Various blends of these qualities are communicated in various tissues. Despite the fact that on a basic level a huge number of kinds of triple-stranded collagen atoms could be gathered from different blends of the 42 o chains, just a set number of triple-helical mixes are conceivable, and under 40 sorts of collagen particles have been found.
Type I
Is by a wide margin the most well-known, being the important collagen of skin and bone. It has a place with the class of fibrillar collagens, or fibril-shaping collagens, which have long ropelike structures with not many or no interferences.
Subsequent to being emitted into the extracellular space, these collagen atoms collect into higher-request polymers called collagen fibrils, which are slight structures. Collagen fibrils regularly total into bigger, link like packages, a few micrometers in breadth called as collagen strands.
Collagen types IX and XII
are called fibril-related collagens since they beautify the outside of collagen fibrils. They are thought to connect these fibrils to each other and to different parts in the extracellular grid.
Type IV is a system shaping collagen, framing a significant piece of basal laminae, while type VII particles structure dimers that collect into particular structures called tying down fibrils. Securing fibrils help append the basal lamina of multilayered epithelia to the basic connective tissue and along these lines are particularly bottomless in the skin.
There are likewise various “collagen-like” proteins, including type XVII which has a transmembrane space and is found in hemi desmosomes, and type XVIII, which is the center protein of a proteoglycan in basal laminae.
The one of a kind properties of each sort of collagen are expected fundamentally to contrasts in (1) the number and lengths of the collagenous, triple-helical portions; (2) the sections that flank or intrude on the triplehelical fragments and that overlap into different sorts of three-dimensional structures; and (3) the covalent adjustment of the alpha chains. Capacities: Collagen filaments, which give mechanical quality and strength
In scuruy, the frequently deadly sickness brought about by a dietary insufficiency of nutrient c that was normal in mariners until the nineteenth century, the damaged professional alpha chains that are integrated neglect to shape a steady triple helix and are quickly corrupted inside the cell, and amalgamation of new collagen is restrained.
In sound tissues, collagen is ceaselessly debased and supplanted (with a turnover time of months or years, contingent upon the tissue). In scurvy, substitution comes up short, and inside a couple of months, with the steady loss of the prior ordinary collagen in the framework, veins become delicate, teeth gotten free in their attachments, and wounds stop to mend.
Elastin (Non-Collagen Component).
Numerous vertebrate tissues, for example, skin, veins, and lungs, should be both solid and flexible so as to work.
A system of flexible filaments in the extracellular grid of these tissues gives them the necessary flexibility with the goal that they can pull back after transient stretch. Versatile strands are in any event multiple times more extensible than an elastic band of a similar cross-sectional territory.
Long, inelastic collagen fibrils are entwined with the flexible strands to constrain the degree of extending and keep the tissue from tearing.
The primary part of flexible filaments is elastin, an exceptionally hydrophobic protein (around 750 amino acids long), which, similar to collagen, is strangely wealthy in proline and glycine be that as it may, in contrast to collagen, isn’t glycosylated and contains some hydroxyproline however no hydroxylysine.
The elastin protein is made to a great extent out of two kinds of short sections that substitute along the polypeptide chain: hydrophobic portions, which are answerable for the flexible properties of the atom; and alanine-and lysinerich a-helical fragments, which structure cross-connects between nearby particles.
Capacities:
Elastin is the prevailing extracellular network protein in courses, including half of the dry load of the biggest supply route the aorta. Changes in the elastin quality causing a lack of the protein in mice or people bring about narrowing of the aorta or different veins and inordinate multiplication of smooth muscle cells in the blood vessel divider. Clearly, the typical versatility of a conduit is required to limit the expansion of these cells.
They help to hold the focal point in its place in the eye. In influenced creatures cause the dislodging of the focal point.
Changes in the fibrillin quality outcome in Marfan’s disorder, a moderately normal human hereditary issue.
Influenced people are regularly strangely tall and lean and have anomalies of the skeleton and joints.
Fibronectin (Non-Collagen Component)
It is an enormous glycoprotein found in all vertebrates and significant for some cell-lattice communications. Fibronectin is a dimer made out of two exceptionally enormous subunits joined by disulfide bonds toward one side.
Every subunit is collapsed into a progression of practically particular areas isolated by districts of adaptable polypeptide chain. The areas thus comprise of littler modules, every one of which are sequentially rehashed and normally encoded by a different exon, recommending that the fibronectin quality, similar to the collagen qualities, advanced by numerous exon duplications.
In the human genome, there is only one fibronectin quality, containing around 50 exons of comparative size, however the transcripts can be joined in various manners to deliver a wide range of fibronectin isoforms Functions:
The freak mice that can’t make fibronectin pass on ahead of schedule in embryogenesis on the grounds that their endothelial cells are neglect to shape appropriate veins.
The deformity is thought to result from anomalies in the collaborations of these cells with the encompassing extracellular network, which regularly contains fibronectin.
http://feeds.feedburner.com/ecarepk