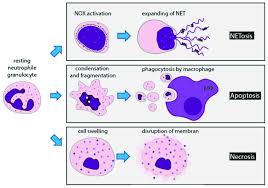
Apoptosis is totally different from tissue rot brought about by an intense physical issue, where cells swell and burst (from osmotic weight contrasts) and cause a critical safe reaction. Apoptosis comprises of 4 stages:
1. The choice to initiate the pathway;
2. The really “self destruction” of the cell;
3. Engulfment of the phone stays by particular resistant cells called phagocytes;
4. Degradation of inundated cell.
The genuine strides in cell demise require:
5. Condensing of the cell core and breaking it into pieces
6. Condensing and dividing of cytoplasm into film bound apoptotic bodies; and Breaking chromosomes into sections containing numerous number of nucleosomes (a nucleosome stepping stool)
7. To end it all must be a critical cell choice. Thus you would anticipate that this cycle should be controlled and profoundly confounded. When might it be profitable to the life form to need a cell to execute itself (or be advised to murder itself)? Cell passing would be utilized to:
8. “sculpt” a living being during improvement, for example, during undeveloped organism advancement, transformation and tissue decay
9. Regulate the complete number of cells.
10. Defend and eliminate undesirable or perilous cells like tumor cells, virally contaminated cells, or insusceptible cells that perceive self (which coujd lead to immune system infection).
Unregulated apoptosis could worsen or cause ailment, for example, AIDS, in which T assistant cell numbers plunge. Part of the emotional decrease in these cells may be brought about by wellbeing T partner cells being fooled into ending it all; neurodegenerative ailments like Alzheimer; ischemic stroke, when confined blood stream to specific districts of the mind can prompt neural demise through expanded apoptosis’ malignancy, in which tumor cells lose their capacity to go through apoptosis; immune system illness, in which self-responsive safe cells stunt typical body cells to execute themselves;
VIRAL DISEASE
Apoptosis doesn’t need new record or interpretation, recommending that the atomic hardware needed for cell passing lay torpid in the cell, and just requires suitable initiation. What “signals” prompt apoptosis? Signs can be extracellular:
1. A hormone, (for example, thyroxin which causes apoptosis in fledgling tails
2. A absence of a “endurance” signal (which restrains apoptosis, for example, a development factor
3. A cell: cell contact from a contiguous cell
4. Signals can be intracellular:
5. Ionizing radiation
6. Virus disease
7. Oxidative harm from free extremists
Systems and Regulation of Apoptosis: Caspases
Portrayal of apoptotic systems and cell players began with the investigation of C. elegens, a round worm. The develop worm has around 1000 cells. During advancement, 131 cells bite the dust. Two transformations were found in which the 131 cells didn’t kick the bucket.
These changes were called ced3 and ced4 (ced represents cell passing). The grouping of ced 3 was extremely homologous to a protein called interleukin changing over compound (ICE) which is needed for proteolytic actuation of the forerunner to interleukin 1, a protein hormone delivered by certain insusceptible cells during enactment and which can advance aggravation.
This recommended that proteolysis was needed for apoptosis. Ensuing examinations show that an entire group of proteases (around 10 in people) called caspases (ICE has been renamed caspase 1) are needed for customized cell demise. These proteases are found in the cell in an idle structure which must go through restricted proteolysis for initiation.
These caspases structure a course of proteases which are enacted in this cycle. They are endo proteases have a functioning site Cys (C) and divide at the C-terminal side of Asp buildups (asp) and thus are known as caspases – cys containing-asp explicit proteases).
ICE isn’t typically engaged with apoptosis, however its fake initiation in refined mammalian cells can prompt it. Every caspase had a similar arrangement as they are intended to sever, so it became apparent that they likely divide each other in an enactment course instrument, like the coagulation protease course of initiation of antecedents (zymogens) of serine proteases which actuate the following in the arrangement. Two arrangement of caspases appear to be included.
One set starts the cycle of caspase enactment. Similarly as in the thickening framework, the subject of what actuates the principal caspase seemed hazardous until specialists found that the initiator caspase can be enacted on the off chance that they total to a basic focus.
This could happen by authoritative of a self destruction signal particle at the phone surface. Conformational changes in the receptor can prompt total of surface receptor atoms with attendant total of intracellular caspases which connect with the accumulated receptors.
Intracellular Signals
In what manner may intracellular activators of apoptosis (like radiation or responsive oxygen species) work. Examination demonstrated association of mitochondria in the apoptotic pathway. In all honesty, cytochrome C, the hemi protein which goes about as a water dissolvable versatile transporter of electrons in mitochondrial oxidative phosphorylation, moving electrons through cytochrome C oxidase or complex IV, spills out of the intermembrane space and ties to a cytoplasmic protein called Apaf-1 for apoptotic protease enacting factor-1. This at that point enacts an initiator caspase-9 in the cytoplasm.
These proteins appear to spill out of mitochondria after a breakdown of the electrochemical potential over the inward film. The expected implodes as an outcome of the kickoff of a channel called a vague internal film porousness progress pore, made out of both an inward layer protein (adenine nucleotide translocator – subterranean insect) and an external layer proteins (porin, the voltage-gated anion channel – VDAC).
These proteins demonstration together, presumably at locales where the internal and external films are in contact. This channel passes anything more modest than atomic weight 1500. Falling the proton angle uncouples oxidation and phosphorylation in the mitochondria. Changes in ionic quality causes an expanding of the grid. Since the internal film is exceptionally tangled and has an a lot more noteworthy surface zone than the external layer, growing of the network prompts a burst of the external layer, spilling the inward film space proteins (cytochrome C and Apaf-1) into the cytoplasm.
MITOCHONDRIA CHANGES DURING APOPTOSIS.
What causes every one of these adjustments in the mitochondria? A few interrelated functions seem, by all accounts, to be included:
1. Disruption of oxphos. Furthermore, electron transport, brought about by illumination and certain second couriers, for example, ceramide.
2. Changes in cell redox potential and age of responsive oxygen species.
3. Damage to DNA (brought about by radiation, roos, and so on) A protein called p53 is regularly communicated in cells with DNA harm. Articulation of this protein brings about restraint of cell division, or apoptosis, the two of which would shield the harmed cell from turning into a tumor cell. Thus the p53 quality is a tumor silencer quality.
It is inactivated by change in around half of all human tumor cells examined. p53 can prompt quality articulation. Of the 14 unique qualities whose articulation are essentially modified by p53, many appear to be utilized by cells to create or react to oxidative pressure. Cells go through p53 apoptosis through oxidative harm.
4. Increases in intracellular calcium particles through sign transduction.
CASPASE TARGETS
Apoptosis includes.
1. Condensing of the cell core and breaking into pieces
2. Condensing and dividing of cytoplasm into layer bound apoptotic bodies
3. Breaking chromosomes into pieces containing different number of nucleosomes (a nucleosome stepping stool)
How does caspase initiation lead to these functions? A protein has been revealed which when cut by a caspase prompts atomic separation. The objective protein is generally bound to another protein, a DNA endonuclease. At the point when the objective protein is divided, the DNase is allowed to relocate to the core and start the execution.
Layer changes in apoptosis happen when caspase 3 divides gelsolin, a protein engaged with keeping up cell morphology. The cut gelsolin separates actin fibers inside the cell. Another protein is important to shape apopotic bodies: a kinase named p21-initiated kinase 2 (PAK-2). This kinase is enacted by caspase-3 by restricted proteolysis. Caspases likewise divide beta-amyloid forerunner protein which may produce more beta-amyloid protein, causing neural cell demise in Alzheimer patients.
CONTROLLING APOPTOSIS
It ought to be certain that cells keep a tight control on the caspases. Two players which seem to repress apoptosis are the mitochondrial proteins Bcl-2 and Bcl-X, which can impede the arrival of cytochrome C from the mitochondria. The Bcl group of proteins have a hydrophobic tail and tie to the external surface of mitochondria and different organelles like the core and endoplasmic reticulum.
These proteins appear to be to have the option to frame particle directs in liposomes. So far 15 individuals from this family (identified with ced-9 of C. elegens) have been found in people. Bcl-2 can likewise tie to Apaf-1 (referenced above) and repress its actuation of initiator caspase-9. Bcl-2 is controlled by changes in the statement of the Bcl-2 quality, by post-translational phosphorylation by kinases, or by cleavage by caspases. Overexpression of Bcl-2 can make a cell become a tumor cell. Another individual from the family, BAX and BAD tie to mitochondria and encourage apoptosis by invigorating cytochrome C discharge.
Also, different proteins called IAP’s (inhibitors of apoptosis) can hinder caspase or other apoptotic proteins. Some infection make the protease to keep their host cells feasible.
CELL MEMBRANE EVENTS
Cells can be told to go through apoptosis through cell surface communications with different cells which are frequently insusceptible cells. One of the positions of the insusceptible cell is to pulverize a modified cell (for instance a virally-tainted.
In one, an actuated lymphocyte ties to an objective cell (like a virally tainted cell) and discharge perforin, a protein which collect in the objective cell layer to frame a transmembrane channel. Different proteins delivered by the actuated lymphocyte can enter the objective cell through the pore and start apoptosis. One such protein that enters, granzyme B is a protease which actuates caspases in the objective cell.
Target cells which express a particular film protein call CD95 (additionally called Fas) are likewise focused for apoptosis. This protein receptor, an individual from the tumor corruption factor receptor (TNFR) ties to a layer protein ligand on the outside of an enacted lympocyte called CD95 Ligand – CD95L-(likewise called the Fas ligand).
On official, the CD95 (Fas) receptors on the objective layer total after compliance changes. A connector protein in the cell, FADD (Fas-related demise area) ties to the amassed cytoplasmic space (the passing space) of CD95 (Fas), and enlisted people dormant caspase-8 to the site, where their fixation increments. This prompts enactment of the caspases.
CD95:CD95L Interactions: (DD is the passing space)
This system is utilized to dispose of initiated lymphocytes after they have completed their work. Actuated invulnerable cells begin communicating Fas a couple of days after initiation, focusing on them for end. A few cells which have been focused on express the two Fas and Fas ligand and execute themselves. Different cells express CD95 (Fas), however CD95L (Fas-Ligand) is communicated predominately by actuated T cells.
Cell surface functions likewise can repress apoptosis. Authoritative of “endurance” factors (like development factors) to cell surface receptors can close of apoptotic pathways in the cells. Some endurance factor receptors are coupled to PI-3-kinase (phosphoinositol-3-kinase) through the G protein ras (p21) which is focused to the cell layer by post-translational expansion of a hydrophobic anchor.
The initiated kinase produces PI-3,4-P2 and PI-3,4,5-P3, which enacts Akt, a Ser/Thr protein kinase. This actuated kinase phosphorylates the proapoptotic-protein BAD, which at that point gets idle. In addition, dynamic Akt phosphorylates prolapse, which in its phosphorylated structure won’t interface with cytochrome C, thus hindering apoptosis.
The endpoint of apoptosis is the engulfment of the divided cell by a phagocytic cell, (for example, a macrophage). In an ongoing article (Nature, 405, pg 85, 2000), it was indicated that the action of phagocytes could be restrained sterospecifically by the expansion of phosphatidyl serine (PS) to the blend, however not by other negative phospholipids.
On the off chance that you recall from our depiction of lipids, PS is found solely in the internal leaflet of red platelets). The agents cloned a quality from the phagocytic cell for a receptor which perceives PS. When added to customary T and B lymphocytes (safe cells), these cells could likewise take-up apoptotic cells. The quality is homologous to qualities in Drosophila (organic product fly) and C. elegens (round worm) recommending that it is rationed in nature.
The message: when cells go through apoptosis, PS, typically discovered distinctly in the inward leaflet, is presented to the outside. It would then be able to tie to receptors on phagocytic cells to finish the cycle of apoptosis.









This is very informative and educational! Keep up the good work!
Good one
This comment has been removed by the author.
Great man,
Please have a look at my site too Trees Varieties
This is very interesting. I love it