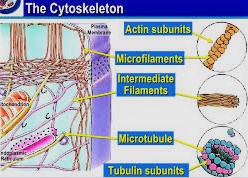
Microtubules, Microfilaments and Intermediate Filaments.
The cytoskeleton is one of a kind to eukaryotic cells. It is a powerful three-dimensional structure that fills the cytoplasm. This structure goes about as both muscle and skeleton, for development and security. The long filaments of the cytoskeleton are polymers of subunits. The essential sorts of strands containing the cytoskeleton are microfilaments, microtubules, and middle fibers.
Microfilaments
Microfilaments
Microfilaments are fine, string like protein filaments, 3-6 nm in distance across. They are made transcendently out of a contractile protein called actin, which is the most plentiful cell protein.
Microfilaments‘ relationship with the protein myosin is answerable for muscle compression. Microfilaments can likewise do cell developments including skimming, withdrawal, and cytokinesis.
Microfilaments‘ relationship with the protein myosin is answerable for muscle compression. Microfilaments can likewise do cell developments including skimming, withdrawal, and cytokinesis.
Actin Filaments .The actin subunit is a solitary globular polypeptide chain and is in this manner a monomer as opposed to a dimer. Every actin fiber has a coupling site for ATP. The actin subunits are gathered head-to-tail to produce fiber with an unmistakable basic extremity.
The actin fiber can be considered to comprise of two equal protofilaments that turn around one another in a privilege gave helix. The auxiliary extremity of actin fibers is made by the standard equal direction of every one of their subunits.
Medications that influences the actin fiber Phallodin: Binds and balance out fibers Cytochalasin: Caps fiber in addition to end Swinholide: cuts off fibers Latrunculin: tie subunit and forestall their polymerization.
The Dynamics of Actin Assembly In a fundamentally polar fiber the active rate steady for affiliation and separation Kon and Koff separately are regularly a lot more noteworthy toward one side than the opposite end. Along these lines if an overabundance of cleansed subunits is permitted to collect onto stamped pieces of performed fibers one finish of each part extends a lot quicker than the other.
In the event that fibers are quickly weakened with the goal that the free subunit fixation dips under the basic focus the quickly developing end likewise depolymerizes quickest. The more powerful of the two finish of a fiber where both development and shrinkage are quick is known as the in addition to end and the opposite end is called as the short end.
Polymerization of G-actin in vitro In the underlying nucleation stage, ATP–G-actin monomers gradually structure stable edifices of actin. These cores are quickly lengthened in the second stage by the expansion of subunits to the two parts of the bargains. In the third stage, the parts of the bargains are in a consistent state with monomeric ATP–G-actin. After their joining into a fiber, subunits gradually hydrolyze ATP and become stable ADP–Factin.
The ATP-restricting clefts of the considerable number of subunits are arranged a similar way in F-actin. Time course of the in vitro polymerization response uncovers the underlying slack time frame. In the event that some actin fiber pieces are included toward the beginning of the response to go about as cores, extension continues quickly with no slack period.
Myosin is an engine protein. The type of myosin found in muscle and first distinguished was myosin-II. Every myosin-II atom is an oligomer made out of one sets of indistinguishable substantial chains and two sets of nonidentical light chains.
Every one of the overwhelming chain has a globular head area at its N-end that contains an actin restricting site and a reactant site that hydrolyzes ATP. Globular head area is trailed by an extremely long amino corrosive arrangement that shapes an all-inclusive curl that intervene overwhelming chain dimerization.
The two light chains near N-terminal head space. Every myosin head ties and hydrolyses ATP, utilizing the vitality of ATP hydrolysis to stroll towards the in addition to end of the actin fiber.
The since quite a while ago snaked followed pack itself with the tail of other myosin particles. These tail-tail connections bring about the arrangement of the enormous bipolar thick fiber that have a few hundred heads situated inverse way at the two finish of the thick fiber.
At the point when myosin is treated with the protease trypsin it is cut into two parts called light meromyosin and substantial meromyosin. Light meromyosin comprises of the majority of the alpha helical rode of the myosin atoms. Overwhelming meromyosin comprises of globular heads with light chain and the rest of the pole area. Consequent treatment of overwhelming meromyosin with papain discharges two globular protein heads called S1 subfragment and a bar like area called S2 subfragment.
The myosin includes in transport of layer vesicles and organelle along actin fiber, phagocytosis and expansion of pseudopods in amoebae.
Microtubules
Microtubules are tube shaped cylinders, 20-25 nm in distance across. They are made out of subunits of the protein tubulin- – these subunits are named alpha and beta. Microtubules go about as a platform to decide cell shape, and give a lot of “tracks” for cell organelles and vesicles to proceed onward.
Microtubules likewise structure the shaft filaments for isolating chromosomes during mitosis. At the point when masterminded in geometric examples inside flagella and cilia, they are utilized for movement.
The essential structure square unit of microtubules is the tubulin that made out of two subunit the α-tubulin and β-tubulin. Countless extra cell particles called microtubule related proteins (MAP) tie to the outside of the microtubule and decide and control its numerous capacities. MAPs assume basic job in the guideline of microtubule polymerization and elements, in the association of microtubule exhibits and in the utilitarian connections of the microtubules with other cell parts.
Microtubule Structure Microtubule polymers are made out of the αβ – tubulin heterodimers orchestrated in a consistently rehashed
Head-to-tail style. The entirety of the β-tubulin subunits face one finish of the microtubules and the α-tubulin subunits face the furthest edge. The finishes are assigned either give or take dependent on their diverse dynamic and auxiliary properties.
The β-subunits are uncovered at the in addition to end while the α-subunits are uncovered at the less closures. Each direct cluster of tubulin heterodimer is known as a protofilament. Microtubule comprises of 13 protofilaments orchestrated into a cylinder structure.
Microtubule in a cell are typically composed at the microtubule arranging focus (MTOC). In creature cells MTOC contain a couple of centrioles and called as centromere. The less finishes are installed at MTOC and the in addition to end are away from it.
One of the known proteins of the MTOC is a type of tubulin called Υtubulin which doesn’t shape microtubule itself and doesn’t get joined into microtubule yet appears to work in the nucleation of microtubule development.
Single microtubule in cytosol is normal yet many particular microtubule exhibits likewise exists in which the microtubules are combined along their lengths into doublet or triplet microtubule structure. Doublet microtubules comprise of 10 or 11 protofilaments of second microtubule intertwined to whole 13 protofilaments first microtubules and triplet microtubules comprise of an extra 10 or 11 protofilaments of third microtubule melded to second microtubules.
Doublet microtubule happens in the long shaft of the motile cell members called cilia and flagella. Triplet microtubule happens in the basal bodies structure arranged at the base of cilia and flagella and in centre.
Microtubule Polymerization and Dynamics Microtubule polymerization happens by nucleation extension instrument in which the development of a short microtubule core is trailed by the development of microtubule at the two its closures by the reversible non-covalent expansion of tubulin subunits. Entire course of polymerization is separated into three stage:
Slack Phase: The slack stage relate to the time taken for nucleation.
Development Phase: The development stage happens as monomers add to the uncovered parts of the bargains fibers causing fibers lengthening.
Consistent Phase: The consistent stage is arrived at when the development of the fiber because of monomer expansion is actually adjusted by the depolymerization from the finishes. The quantity of monomer that adds to the polymer every second relies on the convergence of the free subunits. The centralization of free subunits left at consistent stage point is called as the basic focus.
The pace of the subunit expansion toward the finish of a fiber is the result of the free subunit focus. The pace of polymerization just as depolymerization is a lot quicker for the in addition to end of the fiber than for the short end. When the αβ-tubulin focus is high than the basic fixation at the in addition to end however lower than the basic fixation at the short end, microtubule can treadmill by adding subunits to one end and separating subunits from furthest edge.
This uncommon state in polymer elements is called as the treadmilling keeps the polymer length unaltered. A subsequent conduct is called dynamic flimsiness which is described by the stage changes or exchanging between periods of moderately moderate developing and quick shortening toward the finish of individual microtubule.
Medications that influences microtubules.
Taxol:Binds and balanced out microtubules.
Colchicine, Coleemid: Binds subunit and forestall their polymerization .
Vinblastine, Vincristine: Binds subunit and forestall their polymerization.
Nocodazole: Binds subunit and forestall their polymerization.
Microtubule Motor Proteins: Kinesin and Dynein Kinesin is an engine protein that moves along microtubule. Basically it have two substantial chains and two light chains for each dynamic engine, two globular head area and a stretched wound loop liable for the overwhelming chain dimerization. Huge numbers of the kinesin superfamily individuals have explicit job in the mitotic and meiotic axle development and chromosome detachment during cell division.
http://feeds.feedburner.com/ecarepk