Calcium Concentration Regulation
The resting grouping of Ca2+ in the cytoplasm is regularly kept up around 100 nM, differently announced as 20,000-to 100,000-crease lower than ordinary extracellular fixation. To keep up this low fixation, Ca2+ is effectively siphoned from the cytosol to the extracellular space, the endoplasmic reticulum (ER), and once in a while into the mitochondria. Certain proteins of the cytoplasm and organelles go about as cradles by restricting Ca2+. Flagging happens when the cell is animated to deliver Ca2+ particles from intracellular stores, and additionally when Ca2+ enters the cell through plasma layer particle channels.
Phospholipase C Pathway
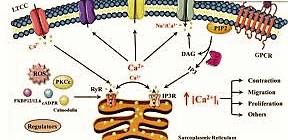
Explicit signs can trigger an unexpected expansion in the cytoplasmic Ca2+ level up to 500–1,000 nM by opening directs in the endoplasmic reticulum or the plasma layer. The most well-known signalin Phospholipase C severing PIP2 into IP3 and DAG g pathway that increments cytoplasmic calcium focus is the phospholipase C pathway.
1. Many cell surface receptors, including G protein-coupled receptors and receptor tyrosine kinases enact the phospholipase C (PLC) compound.
2. PLC hydrolyses the layer phospholipid PIP2 to shape IP3 and diacylglycerol (DAG), two old style second couriers.
3. DAG enlisted people protein kinase C (PKC), joining it to the plasma film
4. IP3 diffuses to the endoplasmic reticulum, and ties to an IP3 receptor,
5. The IP3 receptor fills in as a Ca2+ channel, and deliveries Ca2+ from the endoplasmic reticulum.
6. The Ca2+ particles tie to PKC, enacting it.
Consumption of Ca2+ from the endoplasmic reticulum will prompt Ca2+ section from outside the cell by initiation of “Store-Operated Channels” (SOCs).[4] This inflowing calcium current that outcomes after put away calcium holds have been delivered is alluded to as Ca2+-discharge enacted Ca2+ current (ICRAC).
The systems through which ICRAC happens are right now still under scrutiny, albeit two applicant particles, Orai1 and STIM1, have been connected by a few investigations, and a model of store-worked calcium flood, including these atoms, has been proposed. Late investigations have refered to the phospholipase A2 beta, nicotinic corrosive adenine dinucleotide phosphate (NAADP), and the protein STIM as potential middle people of ICRAC.
Development of Ca2+ particles from the extracellular compartment to the intracellular compartment modifies layer potential. This is found in the heart, during the level period of ventricular compression. In this model, Ca2+ acts to keep up depolarization of the heart. Calcium motioning through particle diverts is additionally significant in neuronal synaptic transmission.
CALCIUM AS A SECOND MESSENGER
Calcium is a pervasive second courier with wide-going physiological jobs. These incorporate muscle compression, neuronal transmission as in an excitatory neurotransmitter, cell motility (counting the development of flagella and cilia), treatment, cell development or multiplication, neurogenesis, learning and memory similarly as with synaptic versatility, and emission of spit. Elevated levels of cytoplasmic Ca2+ can likewise make the cell go through apoptosis.[10] Other biochemical functions of calcium incorporate controlling catalyst action, penetrability of particle channels,[11] movement of particle siphons, and segments of the cytoskeleton.
Huge numbers of Ca2+-intervened functions happen when the delivered Ca2+ ties to and actuates the administrative protein calmodulin. Calmodulin may actuate Ca2+-calmodulin-subordinate protein kinases, or may act straightforwardly on other effector proteins.[13] Besides calmodulin, there are numerous other Ca2+-restricting proteins that intervene the natural impacts of Ca2+.
In neurons, associative expansions in cytosolic and mitochondrial Ca2+ are significant for the synchronization of neuronal electrical movement with mitochondrial energy digestion. Mitochondrial network Ca2+ levels can arrive at the several micromolar levels, which is vital for the initiation of isocitrate dehydrogenase, one of the key administrative proteins of the Krebs cycle.
In neurons, the ER may serve in a network incorporating various extracellular and intracellular signs in a twofold film framework with the plasma layer. Such a relationship with the plasma film makes the moderately new view of the ER and subject of “a neuron inside a neuron.” The ER’s basic qualities, capacity to go about as a Ca2+ sink, and explicit Ca2+ delivering proteins, serve to make a framework that may create regenerative rushes of Ca2+ discharge that may impart both locally and around the world in the phone.
These Ca2+ signals, incorporating extracellular and intracellular transitions, have been ensnared to assume functions in synaptic versatility and memory, synapse discharge, neuronal volatility and long haul changes at the quality record level. Trama center pressure is likewise identified with Ca2+ flagging and alongside the unfurled protein reaction, can cause ER related corruption (ERAD) and autophagy.
CALMODULIN
Calmodulin (CaM) (a contraction for calcium-adjusted protein) is a multifunctional moderate calcium-restricting courier protein communicated in every single eukaryotic cell. It is an intracellular objective of the optional courier Ca2+, and the official of Ca2+ is needed for the initiation of calmodulin. When bound to Ca2+, calmodulin goes about as a feature of a calcium signal transduction pathwAy by adjusting its cooperations with different objective proteins, for example, kinases or phosphatases.
This pictures shows conformational changes in calmodulin. On the left is calmodulin without calcium and on the privilege is calmodulin with calcium. Destinations that predicament target proteins are demonstrated by red stars
Structure
Calmodulin is a little, exceptionally moderated protein that is 148 amino acids long (16.7 KDa). The protein has two roughly even globular areas each containing a couple of EF-hand themes (the N-and C-space) isolated by an adaptable linker locale for an aggregate of four Ca2+ restricting destinations. Each EF-hand theme permits calmodulin to detect intracellular calcium levels by restricting one Ca2+ particle.
Calcium particle restricting districts are found in the accompanying situations in the succession of amino acids: 21-32, 57-68, 94-105 and 130-141; every locale that calcium ties to is actually 12 amino acids long. These locales are situated between two alpha helices in the EF-hand themes, the initial two districts (21-32 and 57-68) are on one side of the linker area the other two (94-105 and 130-141) are on the opposite side.
Significance of Adaptability in Calmodulin
Calmodulin ties such a wide assortment of target proteins, making it particularly significant for it to have adaptability. In spite of the fact that Calmodulin’s adaptability is more obvious when it is bound to an objective protein, NMR examines have indicated that the linker locale of Calmodulin is adaptable, in any event, when it isn’t bound to an objective protein. Another significant quality of calmodulin that permits it to tie an enormous assortment of target proteins is the conventional state of the non-polar sections in the coupling locales.
Since the non-polar depressions are conventional, they don’t need the objective proteins to have a particular grouping of amino acids permitting a bigger assortment of target proteins to be bound. Together, these two auxiliary attributes of calmodulin permit it to deftly tie target proteins with different shapes and amino corrosive successions. For instance, calmodulin ties both NMDA receptors and potassium directs which contrast long by around 50 amino corrosive deposits.
Comparability To Troponin C
Calmodulin’s structure is fundamentally the same as the structure of troponin C (which is another calcium restricting protein). They are the two individuals from the EFh superfamily. Troponin C, as Calmodulin, has two globular spaces that are associated by a linker district. Notwithstanding,
Troponin C and Calmodulin vary in the length of the linker district; the linker locale of Calmodulin is more modest than that of Troponin C. These surprisingly comparative structures are a case of how the EF hand theme is profoundly rationed in calcium restricting proteins. Despite the fact that they have comparative structures, their capacities are altogether different. Troponin C has a quite certain capacity (to inspire a conformational change in Troponin I) at last causing a compression in skeletal muscles. Calmodulin, advanced to tie a more extensive assortment of target proteins, permitting it to assume a part in numerous physiological functions.
Instrument
Up to four calcium particles are limited by calmodulin through its four EF hand motifs.[5] EF hands gracefully an electronegative climate for particle coordination. After calcium official, hydrophobic methyl bunches from methionine buildups become uncovered on the protein through conformational change. Utilizing both X-Ray and NMR contemplates, researchers had the option to verify that the conformational changes happened in the alpha-helices of the EF theme, which changes the coupling liking for target proteins.
At the point when the alpha helices are opposite to each other, the Calmodulin is in an open adaptation giving it a higher proclivity for target proteins.[9] More explicitly, this conformational change presents hydrophobic surfaces, which can thus tie to Basic Amphiphilic Helices (BAA helices) on the objective protein. These helices contain reciprocal hydrophobic areas. The adaptability of calmodulin’s pivoted area permits the atom to fold over its target.[5] This property permits it to firmly tie to a wide scope of various objective proteins. The C-area of calmodulin has a higher proclivity for calcium than does the N-space.
Dynamic Highlights
The spine adaptability inside calmodulin is critical to its capacity to tie a wide scope of targets Protein areas, associated by inherently cluttered adaptable linker spaces, actuate long-range allostery, or the conformational change of a protein by ligand authoritative to an allosteric site (a site other than the useful site), by means of protein area elements.
General Part in The Body
Calmodulin intercedes numerous pivotal cycles, for example, irritation, digestion, apoptosis, smooth muscle withdrawal, intracellular development, present moment and long haul memory, and the resistant reaction. Calcium partakes in an intracellular flagging framework by going about as a diffusible second courier to the underlying improvements. It does this by restricting different focuses in the cell including countless catalysts, particle channels, aquaporins and different proteins.
Calmodulin is communicated in numerous cell types and can have diverse subcellular areas, including the cytoplasm, inside organelles, or related with the plasma or organelle films, yet it is constantly found intracellularly. A significant number of the proteins that Calmodulin ties can’t tie calcium themselves, and use calmodulin as a calcium sensor and sign transducer.
Calmodulin can likewise utilize the calcium stores in the endoplasmic reticulum, and the sarcoplasmic reticulum. Calmodulin can go through post-translational alterations, for example, phosphorylation, acetylation, methylation and proteolytic cleavage, every one of which can possibly adjust its activities.
Function in Smooth Muscle Constriction
1. Calmodulin assumes a significant function in excitation constriction (EC) coupling and the commencement of the cross-connect cycling in smooth muscle, eventually causing smooth muscle compression. To initiate constriction of smooth muscle, the top of the myosin light chain must be phosphorylated. This phosphorylation is finished by Myosin Light Chain (MLC) Kinase. This MLC Kinase is actuated by a calmodulin when it is limited by calcium, along these lines making smooth muscle constriction subject to the presence of calcium, through the authoritative of calmodulin and enactment of MLC kinase.
2. Another way that calmodulin influences muscle constriction is by controlling the development of Ca2+ across both the cell and sarcoplasmic reticulum films. The Ca2+ channels, for example, the ryanodine receptor of the sarcoplasmic reticulum, can be repressed by calmodulin bound to calcium, along these lines influencing the general degrees of calcium in the cell. Calcium siphons remove calcium from the cytoplasm and additionally store it in the endoplasmic reticulum and this control manages numerous downstream cycles.
3. This is a significant capacity of calmodulin in light of the fact that it in a roundabout way assumes a part in each physiological cycle that is influenced by smooth muscle constriction, for example, absorption and withdrawal of supply routes (which circulates blood and control circulatory strain).
Function in Digestion
Calmodulin assumes a significant function in the initiation of phosphorylase kinase, which at last prompts glucose being cut from glycogen by glycogen phosphorylase. Calmodulin additionally assumes a significant part in lipid digestion by influencing Calcitonin. Calcitonin is a polypeptide hormone that brings down blood Ca2+ levels and initiates G Protein falls that prompts the age of cAMP. The activities of calcitonin can be hindered by restraining the activities of calmodulin, recommending that calmodulin assumes a significant function in the initiation of calcitonin.
Function in Present Moment and Long Haul Memory
Ca2+/calmodulin-subordinate protein kinase II (CaMKII) assumes a urgent part in a kind of synaptic pliancy called long haul potentiation (LTP) and requires the presence of calcium/calmodulin. CaMKII adds to the phosphorylation of an AMPA receptor which builds the affectability of AMPA receptors.Furthermore, research shows that hindering CaMKII meddles with LTP.
Function in Plants
Sorghum plant contains temperature-responsive qualities. These qualities help the plant adjust in extraordinary climate conditions, for example, hot and dry environmentsThe plant sorghum is settled model living being and can adjust in hot and dry conditions. Hence, it is utilized as a model to consider calmodulin’s part in plants. Sorghum, contains seedlings that express a glycine-rich RNA-restricting protein, SbGRBP.
This specific protein can be balanced by utilizing heat as a stressor. Its extraordinary area in the cell core and cytosol, shows cooperation with calmodulin that requires the utilization of Ca2+. By presenting the plant to flexible pressure conditions, it can cause various proteins that empower the plant cells to endure ecological changes to get subdued.
These balanced pressure proteins are appeared to associate with CaM. In an Arabidopsis thaliana study, many various proteins showed the likelihood to tie to CaM in plants. The CaMBP qualities communicated in the sorghum are portrayed as a “model harvest” for exploring the resilience to warmth and dry spell pressure.