Electrophoresis.
Is the development of dissipated particles relative with a fluid influenced by a spatially uniform electric field. This electrokinetic wonder was viewed unprecedented for 1807 by Reuss, who saw that the usage of a steady electric field caused earth particles dissipated in water to move. It is in the long run achieved by the proximity of a charged interface between the atom surface and the incorporating fluid.
Standard.
The dispersed particles have an electric surface charge, on which an outside electric field applies an electrostatic Coulomb power. As showed by the twofold layer theory, each and every surface charge in fluids are screened by a diffuse layer of particles, which has a comparative out and out blame yet opposite sign for respect to that of the surface charge.
The electric field moreover applies a force on the particles in the diffuse layer which has going to that following up on a shallow level charge. This last force isn’t generally applied to the atom, anyway to the particles in the diffuse layer arranged at some great ways from the particle surface, and some bit of it is moved right to the atom surface through thick weight. This bit of the force is in like manner called electrophoretic deterrent force.
Gel Electrophoresis.
In the previous occasions, DNA pieces were troublesomely disengaged by using gravity. During the 1970s, the helpful resource called DNA gel electrophoresis was made, in which force was used to disengage DNA pieces by size as they move through a penetrable gel framework.Gel electrophoresis is by and by used to filter through strands of DNA, RNA or protein particles by size, by using agarose gel and electrical stream.
This is cultivated by moving unfavorably charged DNA particles through an agarose structure to which an electric field is applied (electrophoresis).Shorter molecules move speedier and migrate more far off than the more drawn out ones, right now them by size. DNA Gel electrophoresis is generally used after upgrade of DNA using PCR framework. It is done in the going with progresses:
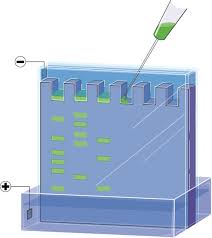
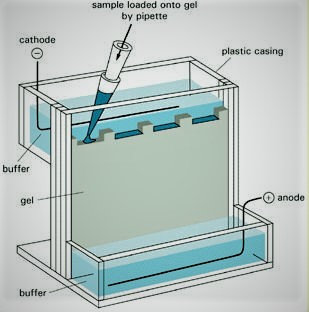
To make agarose gel, put a little agarose into a carafe and include support answer for it. The support causes the electric stream to experience the gel.Warmth the course of action until agarose condenses in the pad and a short time later discharges the material into the gel structure. Detect the brush in structure to make wells in the gel in which DNA can later be put.
At the moment that the gel solidifies, warily oust the brush. Void help into the electrophoresis box. The pad drives the electric stream and shields the gel from drying out. Recognize the gel shape inside the electrophoresis box, barely submerged in the pad. Incorporate stacking bolster that contains shading to the DNA test. Use micropipette to stack DNA test mixed in with the stacking pad into the essential well of the agarose gel. Use micropipette to stack DNA size standard into the accompanying extraordinary of the agarose gel.
The DNA size standard contains strands of known length. Partner the electrophoresis box to the electric stream. The negative (dim) finish of the current must be put on the end closest to the wells. Turn on the electric stream. The DNA strands move away from the negative current. The short strands travel through the gel more quickly than the long strands.
This can be seen due to the blue shade of the stacking support. Temperament executioner current and oust the gel shape from the electrophoresis box. Spot gel in DNA recoloring game plan, ethidium bromide. This connections to DNA and can be seen under glaring light. Remove gel from ethidium bromide after around 30 minutes and spot it on UV light box. The DNA gatherings will be clear for both the DNA test and DNA size standard.
Precipitation is the course of action of a solid in an answer or inside another solid during a substance reaction or by dispersal in a solid. Right when the reaction occurs in a liquid, the solid confined is known as the quicken, or when compacted by a rotator, a pellet. The liquid remaining over the solid is in either case called the supernate or supernatant. Powders got from precipitation have also undeniably been known as blooms.
Sedimentation,
Where a solid structure over some stretch of time as a result of encompassing forces likes gravity or centrifugation. During creation reactions, precipitation may moreover happen particularly if an insoluble substance is brought into an answer and the thickness happens to be increasingly conspicuous (regardless the quicken would float or structure a suspension). With dissolvable substances, precipitation is animated once the game plan gets supersaturated.
In solids, precipitation occurs if the centralization of one in number is over beyond what many would consider possible in the host solid, due to for instance brisk dousing or molecule implantation, and the temperature is adequately high that scattering can provoke disconnection into accelerates.Precipitation in solids is routinely used to mix nanoclusters.
A critical period of the precipitation technique is the start of nucleation. The creation of a hypothetical solid atom fuses the advancement of an interface, which requires some essentialness reliant on the relative surface imperativeness of the solid and the arrangement.On the remote possibility that this imperativeness isn’t available, and no proper nucleation surface is open, supersaturation occurs.
1. Precipitation.
Responses can be used for making hues, removing salts from water in water treatment, and in old style emotional inorganic assessment.
Precipitation is moreover useful to withdraw the aftereffects of a reaction during workup. Ideally, the consequence of the reaction is insoluble in the reaction dissolvable. Along these lines, it quickens as it is encircled, in a perfect world forming unadulterated valuable stones.A instance of this would be the blend of porphyrins in refluxing propionic destructive. By cooling the reaction mix to room temperature, jewels of the porphyrin hurry, and are assembled by filtration:
Precipitation may moreover happen when an antisolvent (a dissolvable in which the thing is insoluble) is incorporated, and dreied drastically decreasing the dissolvability of the perfect thing. From that point on, the rush may easily be separated by filtration, exhausting, or centrifugation). A model would be the mix of chromic tetraphenylporphyrin chloride: water is added to the DMF reaction course of action, and the thing rushes.
2.Electrophoresis Separation Techniques Applications Precipitation Centrifugation Centrifugation.
Centrifugation is a system that incorporates the use of the emanating power for the division of mixes, used in industry and in look into office settings. Progressively thick pieces of the mix move away from the turn of the rotator, while less-thick fragments of the mix move towards the hub. Physicists and scientist may construct the convincing gravitational force on a test tube so as to even more rapidly and thoroughly aim the energize (“pellet”) to amass on the base of the chamber.
The rest of the plan is suitably called the “supinate” or “supernatant liquid”. The supernatant liquid is then either quickly tapped from the chamber without upsetting the quicken, or pulled back with a Pasteur pipette.
The pace of centrifugation is dictated by the stimulating applied to the model, ordinarily evaluated in cycles each minute (RPM) or g.The particles’ settling speed in centrifugation is a component of their size and shape, diffusive speeding up, the volume division of solids present, the thickness differentiate between the atom and the liquid, and the consistency.
An hub is used to disconnect particles or macromolecules:
- Cells Sub-cell portions Proteins Nucleic acids.
- Reason of division: Size Shape Density
- Theory: Utilizes thickness differentiation between the particles/macromolecules and the medium in which these are dissipated Dispersed structures are presented to misleadingly impelled gravitational fields.
http://feeds.feedburner.com/ecarepk
Post Views:
55